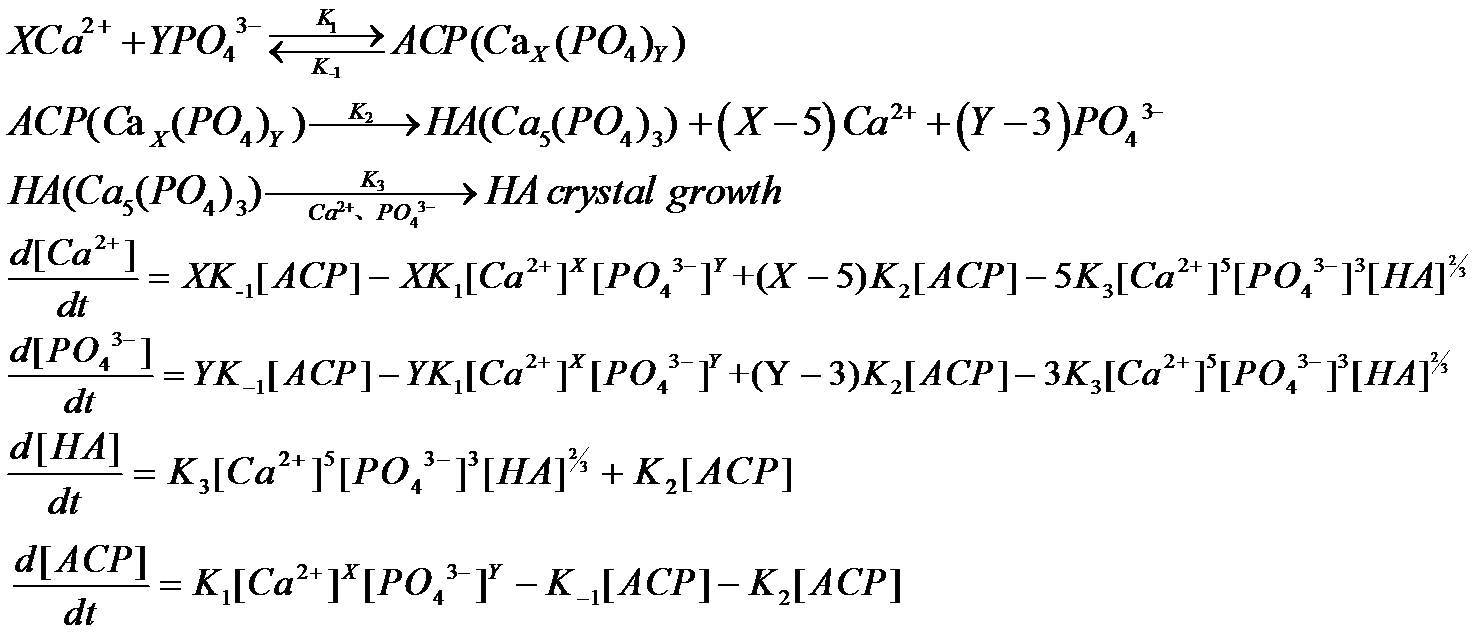IADR Abstract Archives
Stabilizing effect of ionic polymers on calcium phosphate mineralizing solutions: A kinetic study
Objectives: A significant amount of research has shown that in the presence of polymers, such as polyallylamine hydrochloride (PAH), amorphous calcium phosphate (ACP) can be stable in a supersaturated calcium phosphate solution for an appreciable period of time. However, the effect of varying the concentration of the polymer on the kinetics of ACP's transformation to hydroxyapatite (HA) has not been studied in detail. The aim of our study is therefore to study the effect of different concentrations of PAH on the stability of calcium phosphate solutions by developing a kinetic model to predict changes in ion concentrations.
Methods: Mineralizing solutions were prepared by mixing equal volumes of 9 mM CaCl2 and 4.2 mM K2HPO4 solutions, supplemented with 0, 50, 100 and 200 µg/mL of PAH (MW 15 kDa). The mineralizing solutions were placed in an incubator kept at 37 °C. Changes in the concentration of Ca2+ were measured using a pH meter with a Ca2+ electrode. The turbidity of the solutions was measured using a spectrophotometer.
The overall reactions and kinetic equations of the different reactions were assumed to be Fig.1
The concentrations of Ca2+, PO43-, Mg2+, ACP and HA were normalized by the initial Ca2+ concentration. The rate constants (K) were calibrated by fitting the numerical solutions to the measured changes in Ca2+ concentration. The predicted solution turbidity was then compared with the experimental results.
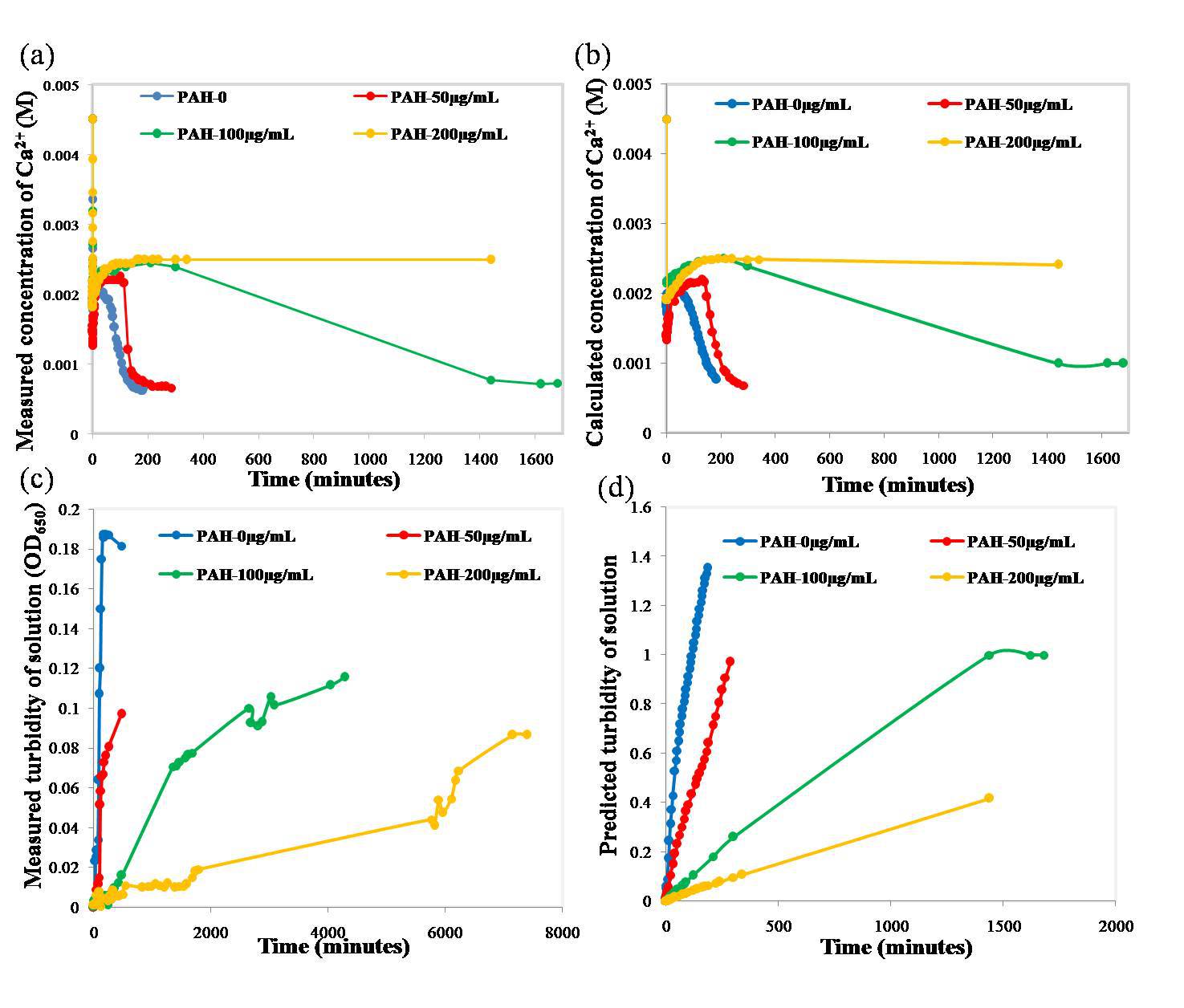
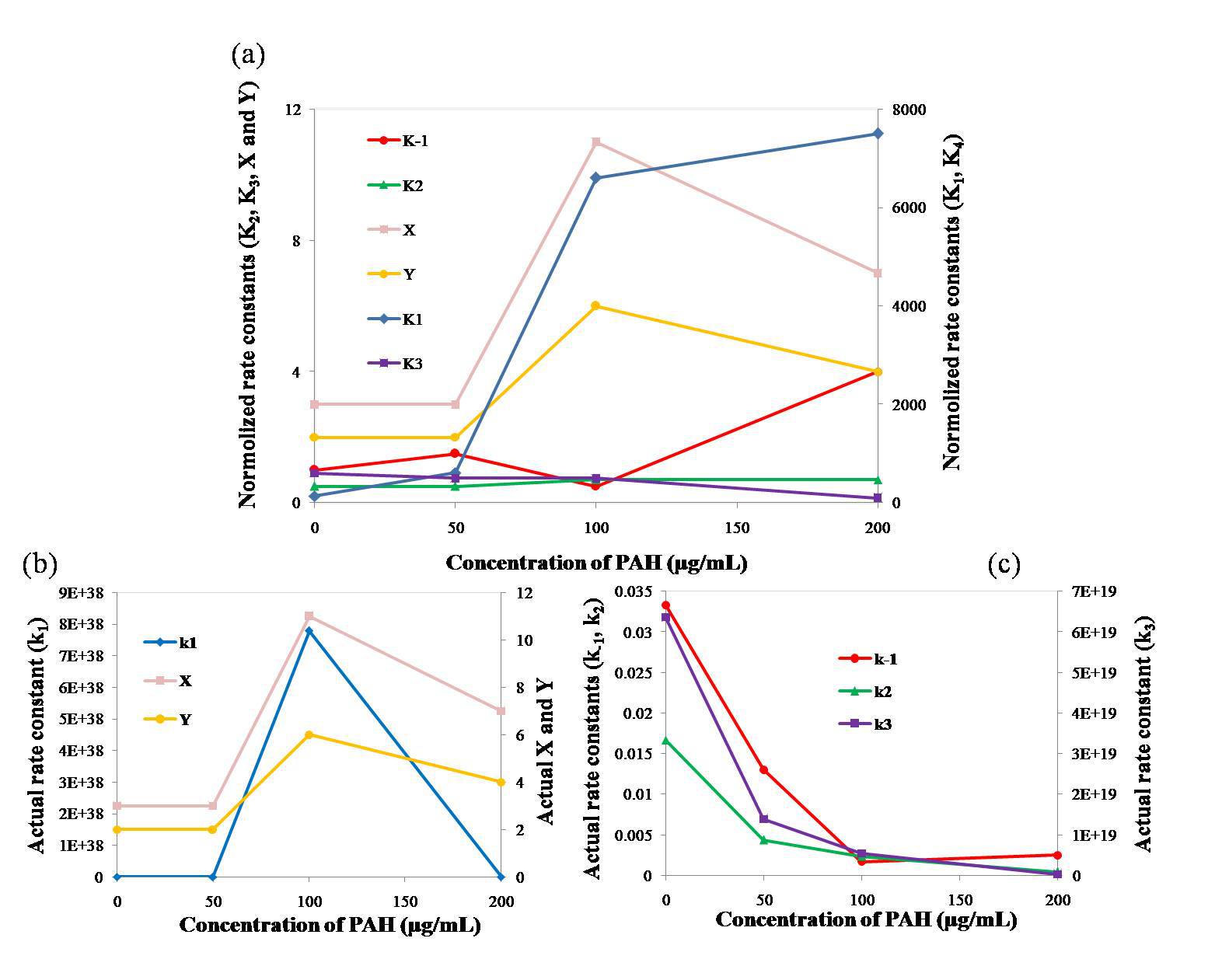
Results: The concentration of Ca2+ in the solutions dropped sharply at the beginning of mixing. After the initial drop, the Ca2+ concentration quickly returned to a higher value, at which it remained for a period of time before decreasing again. The higher the polymer concentration, the longer the period of stability. Numerical solutions of the calibrated rate equations correctly captured the changes in Ca2+ concentration (Fig. 2(a) and (b)). Fig. 3 shows that normolized and actual rate constants. The actual k1, X and Y increased firstly, then decreased. And the actual k-1, k2 and k3 decreased as the PAH concentration increased. The predicted turbidity of the solutions showed similar rates of increase as those measured (Fig. 2(c) and (d)).
Conclusions: A kinetic model has been developed successfully to simulate the concentration-dependent stabilizing effect of PAH on calcium phosphate mineralizing solutions.
Methods: Mineralizing solutions were prepared by mixing equal volumes of 9 mM CaCl2 and 4.2 mM K2HPO4 solutions, supplemented with 0, 50, 100 and 200 µg/mL of PAH (MW 15 kDa). The mineralizing solutions were placed in an incubator kept at 37 °C. Changes in the concentration of Ca2+ were measured using a pH meter with a Ca2+ electrode. The turbidity of the solutions was measured using a spectrophotometer.
The overall reactions and kinetic equations of the different reactions were assumed to be Fig.1
The concentrations of Ca2+, PO43-, Mg2+, ACP and HA were normalized by the initial Ca2+ concentration. The rate constants (K) were calibrated by fitting the numerical solutions to the measured changes in Ca2+ concentration. The predicted solution turbidity was then compared with the experimental results.
Results: The concentration of Ca2+ in the solutions dropped sharply at the beginning of mixing. After the initial drop, the Ca2+ concentration quickly returned to a higher value, at which it remained for a period of time before decreasing again. The higher the polymer concentration, the longer the period of stability. Numerical solutions of the calibrated rate equations correctly captured the changes in Ca2+ concentration (Fig. 2(a) and (b)). Fig. 3 shows that normolized and actual rate constants. The actual k1, X and Y increased firstly, then decreased. And the actual k-1, k2 and k3 decreased as the PAH concentration increased. The predicted turbidity of the solutions showed similar rates of increase as those measured (Fig. 2(c) and (d)).
Conclusions: A kinetic model has been developed successfully to simulate the concentration-dependent stabilizing effect of PAH on calcium phosphate mineralizing solutions.