IADR Abstract Archives
Establishment of primary cell line from oral submucous fibrosis sample: a novel in-vitro preclinical model of fibrosis
Objectives: Oral submucous fibrosis (OSMF) is a chronic oral premalignant condition that vitiates the quality of life of the patient. Contemporarily, the disease is devoid of any definitive treatment and existing management modalities are solely focused on alleviating the symptoms. Therefore, developing a definite and pragmatic in-vitro model at the earliest that could be employed for drug screening is of utmost importance. For achieving this goal, a patient-derived primary cell line can serve as an ideal preclinical model for a debilitating condition like OSMF. The objective of the present study was to establish and characterize the primary cell line of oral fibroblasts from OSMF tissue sample.
Methods: Primary cell culture isolation was performed after obtaining buccal mucosa tissue from the OSMF patient. Afterwards, enzymatic digestion was performed via trypsin and the sample was cultured in Dulbecco modified eagle medium (DMEM) supplemented with fetal bovine serum and penicillin/streptomycin. Oral fibroblasts were observed under the inverted microscope on the fifth day. On achieving 80% confluency, the fibroblasts were trypsinized and subcultured till the sixth passage. Morphological analysis of the fibroblasts was done using live cell imaging microscopy. Population doubling time (PDT) of the cells was analyzed at different passages to assess the growth characteristics. The cell line was characterised through the expression of fibroblasts specific markers (actin, vimentin and CD90) by employing qPCR and immunocytochemistry. Gene expression of the pathological markers (COMP, TGM2 and TIMP) was also assessed and compared with the OSMF tissue sample using qPCR.
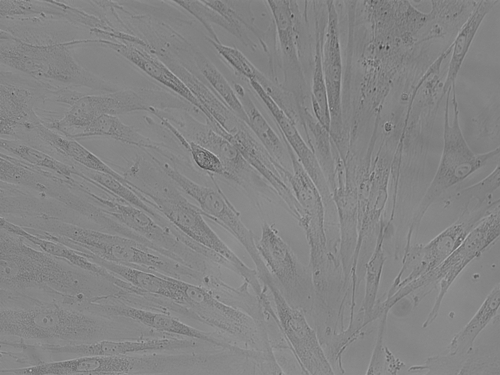
Results: A monolayer of spindle-shaped oral fibroblasts was observed on the 5th day after the initial cell culture. Variation in morphological phenotypes of fibroblasts was observed in the successive passages. The positive expression of fibroblasts specific markers (actin, vimentin and CD90) was reported. Pathological markers (COMP, TGM2 and TIMP) also showed positive expression.
Conclusions: The current study successfully achieved the establishment and characterization of the primary cell line from the OSMF tissue sample. The OSMF cell line would serve as a reliable, safe and convenient in-vitro platform for experimentation for the disease.
Methods: Primary cell culture isolation was performed after obtaining buccal mucosa tissue from the OSMF patient. Afterwards, enzymatic digestion was performed via trypsin and the sample was cultured in Dulbecco modified eagle medium (DMEM) supplemented with fetal bovine serum and penicillin/streptomycin. Oral fibroblasts were observed under the inverted microscope on the fifth day. On achieving 80% confluency, the fibroblasts were trypsinized and subcultured till the sixth passage. Morphological analysis of the fibroblasts was done using live cell imaging microscopy. Population doubling time (PDT) of the cells was analyzed at different passages to assess the growth characteristics. The cell line was characterised through the expression of fibroblasts specific markers (actin, vimentin and CD90) by employing qPCR and immunocytochemistry. Gene expression of the pathological markers (COMP, TGM2 and TIMP) was also assessed and compared with the OSMF tissue sample using qPCR.
Results: A monolayer of spindle-shaped oral fibroblasts was observed on the 5th day after the initial cell culture. Variation in morphological phenotypes of fibroblasts was observed in the successive passages. The positive expression of fibroblasts specific markers (actin, vimentin and CD90) was reported. Pathological markers (COMP, TGM2 and TIMP) also showed positive expression.
Conclusions: The current study successfully achieved the establishment and characterization of the primary cell line from the OSMF tissue sample. The OSMF cell line would serve as a reliable, safe and convenient in-vitro platform for experimentation for the disease.