IADR Abstract Archives
Designing Personalized Treatment for HNSCC Patients.
Objectives: Tremendous molecular heterogeneity between head and neck squamous cell
carcinoma (HNSCC) patients has hindered the development of targeted drugs to successfully
target this cancer type. Although (80–90%) of HNSCC harbor activated EGF Receptor, anti-
EGFR monotherapies did not improve significantly anti-HNSCC treatment. We hypothesize
that existence of additional, patient-specific molecular processes is the main reason for
therapeutic failure. Thus, quantitative strategies allowing to resolve inter-tumor heterogeneity
and then to design patient-specific combined targeted therapies are urgently required.
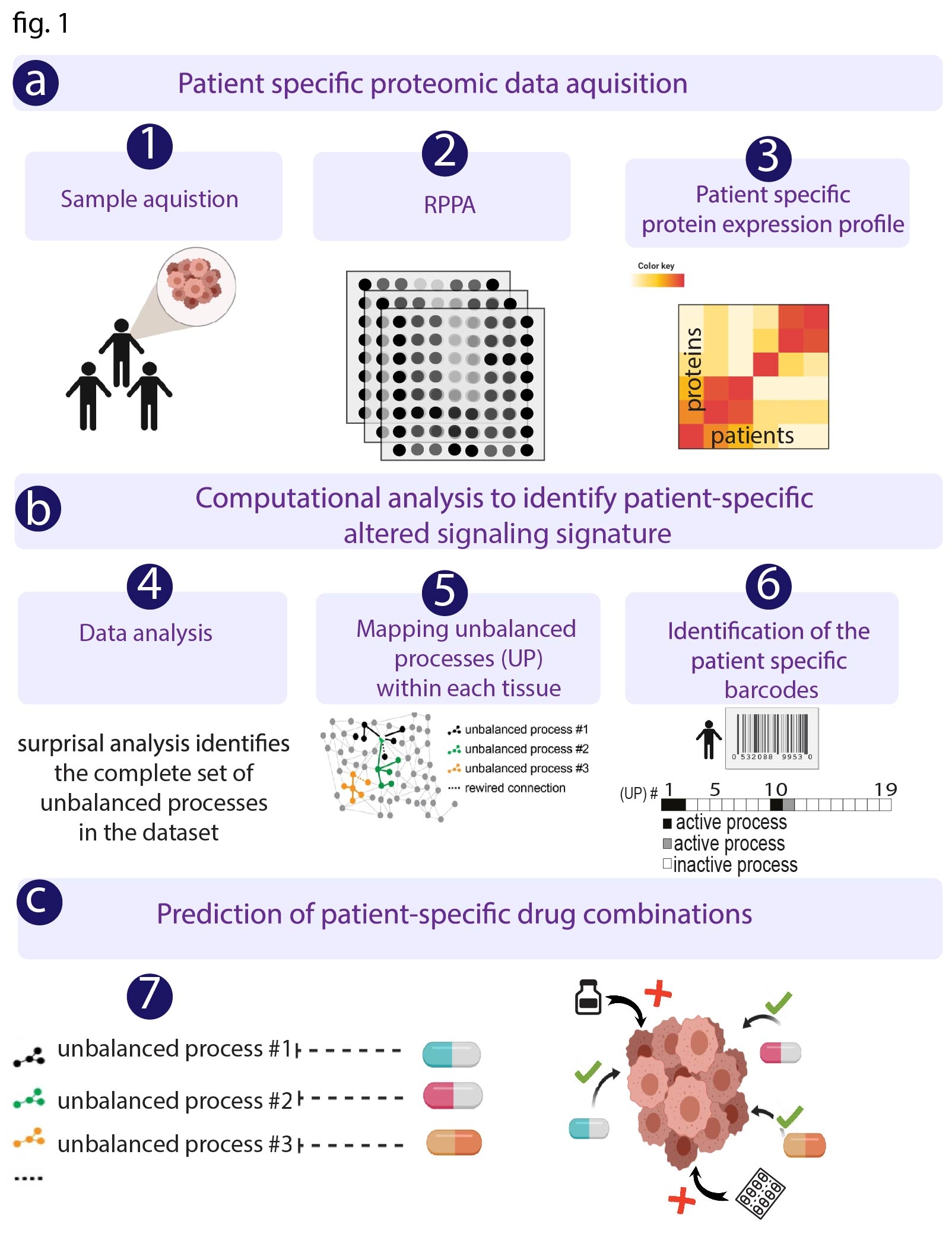
Methods: To address this problem, we analysed large datasets including multiple HNSCC cell
lines (obtained from TCGA-portal) and patient-derived tissues treated with EGFR inhibitors
using computational, information-theoretic approach called Surprisal Analysis (SA). We have
recently extended SA to the field of personalized medicine to allow to resolve individualized,
patient-specific signaling/gene-expression signatures. Each signature may consist of several
altered protein/gene co-expression subnetworks. Based on those signatures personalized
combined targeted treatments are designed. We validated this strategy in-vitro and in-vivo
using two EGFR overexpressing HNSCC cell lines (Cal27 and SCC25), which were
randomly selected from TCGA dataset.
Results: We show that malignancies from the same cancer type (e.g, HNSCC from tongue)
can have different signaling signature and thus require different combined treatments. We
show that the predicted by the analysis combinations are more effective than EGFR
monotherapies. Moreover, they are highly selective: a very efficient combination for one cell
line is significantly less efficient for another cell line and vice versa. Furthermore, we show
that predicted drug combinations induced T-cell immune response, increasing IFλ levels in a
personalized manner.
Conclusions: We show that poor efficiency of EGFR monotherapy can be explained by
existence of additional, patient-specific subnetworks that need to be targeted. We demonstrate
a strategy that transforms high complexity of HNSCC into simple, patient-specific signatures
thereby providing a guidance on how personalized therapies can be rationally designed.
carcinoma (HNSCC) patients has hindered the development of targeted drugs to successfully
target this cancer type. Although (80–90%) of HNSCC harbor activated EGF Receptor, anti-
EGFR monotherapies did not improve significantly anti-HNSCC treatment. We hypothesize
that existence of additional, patient-specific molecular processes is the main reason for
therapeutic failure. Thus, quantitative strategies allowing to resolve inter-tumor heterogeneity
and then to design patient-specific combined targeted therapies are urgently required.
Methods: To address this problem, we analysed large datasets including multiple HNSCC cell
lines (obtained from TCGA-portal) and patient-derived tissues treated with EGFR inhibitors
using computational, information-theoretic approach called Surprisal Analysis (SA). We have
recently extended SA to the field of personalized medicine to allow to resolve individualized,
patient-specific signaling/gene-expression signatures. Each signature may consist of several
altered protein/gene co-expression subnetworks. Based on those signatures personalized
combined targeted treatments are designed. We validated this strategy in-vitro and in-vivo
using two EGFR overexpressing HNSCC cell lines (Cal27 and SCC25), which were
randomly selected from TCGA dataset.
Results: We show that malignancies from the same cancer type (e.g, HNSCC from tongue)
can have different signaling signature and thus require different combined treatments. We
show that the predicted by the analysis combinations are more effective than EGFR
monotherapies. Moreover, they are highly selective: a very efficient combination for one cell
line is significantly less efficient for another cell line and vice versa. Furthermore, we show
that predicted drug combinations induced T-cell immune response, increasing IFλ levels in a
personalized manner.
Conclusions: We show that poor efficiency of EGFR monotherapy can be explained by
existence of additional, patient-specific subnetworks that need to be targeted. We demonstrate
a strategy that transforms high complexity of HNSCC into simple, patient-specific signatures
thereby providing a guidance on how personalized therapies can be rationally designed.