IADR Abstract Archives
Antimicrobial activity of a stannous fluoride toothpaste in the PGRM
Objectives: To assess ex-vivo antimicrobial activity of a non-aqueous toothpaste containing 0.454% stannous fluoride (SnF2) on de-novo plaque in two plaque glycolysis and regrowth models.
Methods: Two single-centre, analyst/examiner blind, randomised, three-treatment studies were conducted in healthy adults with plaque acidity pH 5.0–5.7. Test (0.454% SnF2) and Positive Control (Crest® ProHealth: 0.454% SnF2 and zinc citrate) toothpastes were compared to a Negative Control (regular) toothpaste. Baseline plaque samples were collected from maxillary dentition. Subjects then either brushed maxillary dentition with their assigned toothpaste for 30 seconds and swilled the resulting slurry for 30 seconds (Study 1) or rinsed the whole mouth with a pre-prepared toothpaste slurry for 60 seconds (Study 2). Plaque samples (mandibular, except right maxillary for Study 2, 15 minutes) were collected at 15, 45 (both studies) and 90 minutes (Study 2) and analysed for acidogenicity and regrowth activity. Area-under-the-curve (AUC)regrowth and AUCglycolysis were calculated over 45 (both studies) and 90 minutes (Study 2).
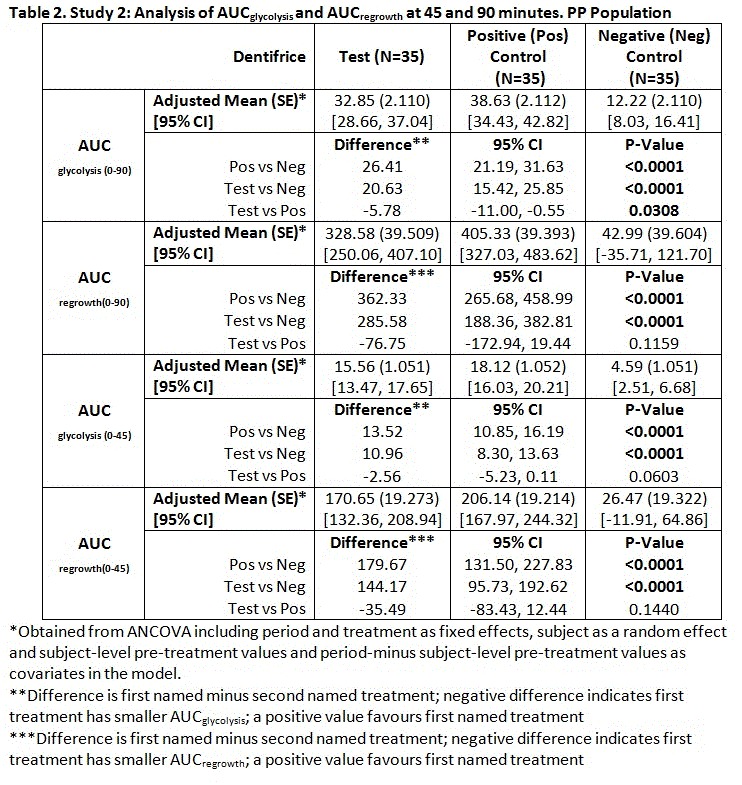
Results: Study 1: Differences in mean AUCglycolysis and AUCregrowth between Positive and Negative Controls weren’t significant, study validity was not achieved (Table 1). Study 2: Significant differences were shown in mean AUCglycolysis and AUCregrowth between Positive and Negative Controls and between Test and Negative Control at both timepoints, favouring the first named treatments. Mean difference in AUCglycolysis(0-90) between Test and Positive Control was statistically significant, favouring the latter, with no significant difference for AUCregrowth(0-90) or for either measure at 45 minutes (Table 2). There were no treatment-related or serious adverse events.
Conclusions: While Study 1 was not validated, Study 2 was. In Study 2, antimicrobial biological activity was demonstrated for the 0.454% SnF2 non-aqueous toothpaste. The difference in AUCglycolysis(0-90) observed for the Positive Control compared to the Test is attributed to the presence of zinc. All study products were well tolerated. Study funded by GSK Consumer Healthcare.
Methods: Two single-centre, analyst/examiner blind, randomised, three-treatment studies were conducted in healthy adults with plaque acidity pH 5.0–5.7. Test (0.454% SnF2) and Positive Control (Crest® ProHealth: 0.454% SnF2 and zinc citrate) toothpastes were compared to a Negative Control (regular) toothpaste. Baseline plaque samples were collected from maxillary dentition. Subjects then either brushed maxillary dentition with their assigned toothpaste for 30 seconds and swilled the resulting slurry for 30 seconds (Study 1) or rinsed the whole mouth with a pre-prepared toothpaste slurry for 60 seconds (Study 2). Plaque samples (mandibular, except right maxillary for Study 2, 15 minutes) were collected at 15, 45 (both studies) and 90 minutes (Study 2) and analysed for acidogenicity and regrowth activity. Area-under-the-curve (AUC)regrowth and AUCglycolysis were calculated over 45 (both studies) and 90 minutes (Study 2).
Results: Study 1: Differences in mean AUCglycolysis and AUCregrowth between Positive and Negative Controls weren’t significant, study validity was not achieved (Table 1). Study 2: Significant differences were shown in mean AUCglycolysis and AUCregrowth between Positive and Negative Controls and between Test and Negative Control at both timepoints, favouring the first named treatments. Mean difference in AUCglycolysis(0-90) between Test and Positive Control was statistically significant, favouring the latter, with no significant difference for AUCregrowth(0-90) or for either measure at 45 minutes (Table 2). There were no treatment-related or serious adverse events.
Conclusions: While Study 1 was not validated, Study 2 was. In Study 2, antimicrobial biological activity was demonstrated for the 0.454% SnF2 non-aqueous toothpaste. The difference in AUCglycolysis(0-90) observed for the Positive Control compared to the Test is attributed to the presence of zinc. All study products were well tolerated. Study funded by GSK Consumer Healthcare.