IADR Abstract Archives
Impact of Catechol Functionalities on Network Properties and Adhesive Performance
Objectives: The impact of non-covalent interactions (e.g., hydrogen bonding, pi-pi stacking) on adhesive network performance were investigated by incorporating varying quantities of catechol-functionalized methacrylates into model adhesive resins.
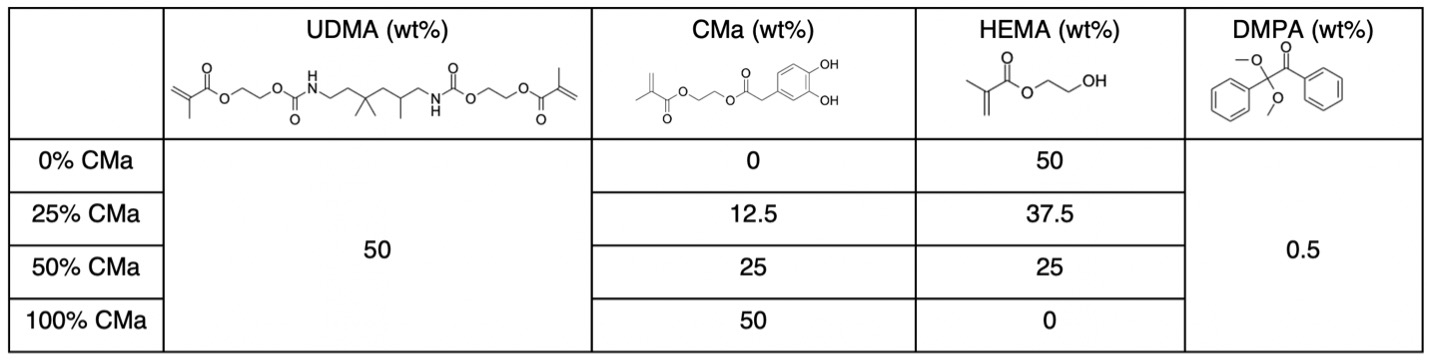
Methods: Catechol methacrylate (CMa) was synthesized using the Steglich reaction. Experimental resins consisted of a cross-linker (UDMA), which was maintained at a level of 50 wt%. In control samples (0% CMa), the remaining 50 wt% of the adhesive resin consisted of HEMA. To explore the impact of catechol functionalities on adhesive performance, HEMA was systematically replaced by CMa (see Table). Fourier-transform infrared spectroscopy (FT-IR), Dynamic mechanical analysis (DMA), water swelling, and adhesive strength were characterized for each formulation.
Results: The resins including CMa showed slightly reduced overall conversion and maximum polymerization rate (Rp) compared to the control. However, the addition of CMa in lieu of HEMA did not significantly hinder the polymerization kinetics of the UDMA/HEMA-based resin, as the 50 wt% CMa resin still had a reasonable overall conversion of ~80%. DMA characterization revealed that network Tg decreases with CMa modification, however the plateau storage modulus (E’) is unchanged. Additionally, the breadth of tan delta peaks observed via DMA are consistent with CMa modification, indicating the novel monomer integrates well into the network. Most notably, water sorption in resins containing 25 and 50 wt% CMa is significantly reduced over a period of 40 days. This is a promising result, as the reduction in water sorption reduces the susceptibility of these networks to methacrylate hydrolysis.
Conclusions: The introduction of catechol-functionalized monomers can improve the performance of adhesive materials by incorporating additional non-covalent interactions within a polymer network. Our work reveals that model adhesive formulations containing CMa had lower water sorption, which may reduce susceptibility of these materials to hydrolytic degradation, a major challenge facing currently employed dental adhesives.
Methods: Catechol methacrylate (CMa) was synthesized using the Steglich reaction. Experimental resins consisted of a cross-linker (UDMA), which was maintained at a level of 50 wt%. In control samples (0% CMa), the remaining 50 wt% of the adhesive resin consisted of HEMA. To explore the impact of catechol functionalities on adhesive performance, HEMA was systematically replaced by CMa (see Table). Fourier-transform infrared spectroscopy (FT-IR), Dynamic mechanical analysis (DMA), water swelling, and adhesive strength were characterized for each formulation.
Results: The resins including CMa showed slightly reduced overall conversion and maximum polymerization rate (Rp) compared to the control. However, the addition of CMa in lieu of HEMA did not significantly hinder the polymerization kinetics of the UDMA/HEMA-based resin, as the 50 wt% CMa resin still had a reasonable overall conversion of ~80%. DMA characterization revealed that network Tg decreases with CMa modification, however the plateau storage modulus (E’) is unchanged. Additionally, the breadth of tan delta peaks observed via DMA are consistent with CMa modification, indicating the novel monomer integrates well into the network. Most notably, water sorption in resins containing 25 and 50 wt% CMa is significantly reduced over a period of 40 days. This is a promising result, as the reduction in water sorption reduces the susceptibility of these networks to methacrylate hydrolysis.
Conclusions: The introduction of catechol-functionalized monomers can improve the performance of adhesive materials by incorporating additional non-covalent interactions within a polymer network. Our work reveals that model adhesive formulations containing CMa had lower water sorption, which may reduce susceptibility of these materials to hydrolytic degradation, a major challenge facing currently employed dental adhesives.