IADR Abstract Archives
Kinetics of Photoinitiated Thiol–Michael Addition Reactions
Objectives: To develop a fundamental mathematical framework that provides new insights about the kinetics of thiol–Michael addition reactions involving photo-cleavable base generators useful for the development of multifunctional photopolymerization systems, and their implementation in dental restorative materials.
Methods: Kinetic studies were conducted by monitoring the progress of the photochemical reaction via time-resolved FT–IR spectroscopy and the observed kinetics modeled both analytically and numerically using a basic mechanistic scheme proposed for the reaction. Rate coefficients intrinsic to the propagation–chain-transfer mechanism, characterizing the anionic cycle, were estimated using the ‘flooding’ technique. Multifunctional photopolymerization systems were modeled using derived rate equations accounting both for the kinetics and diffusional processes.
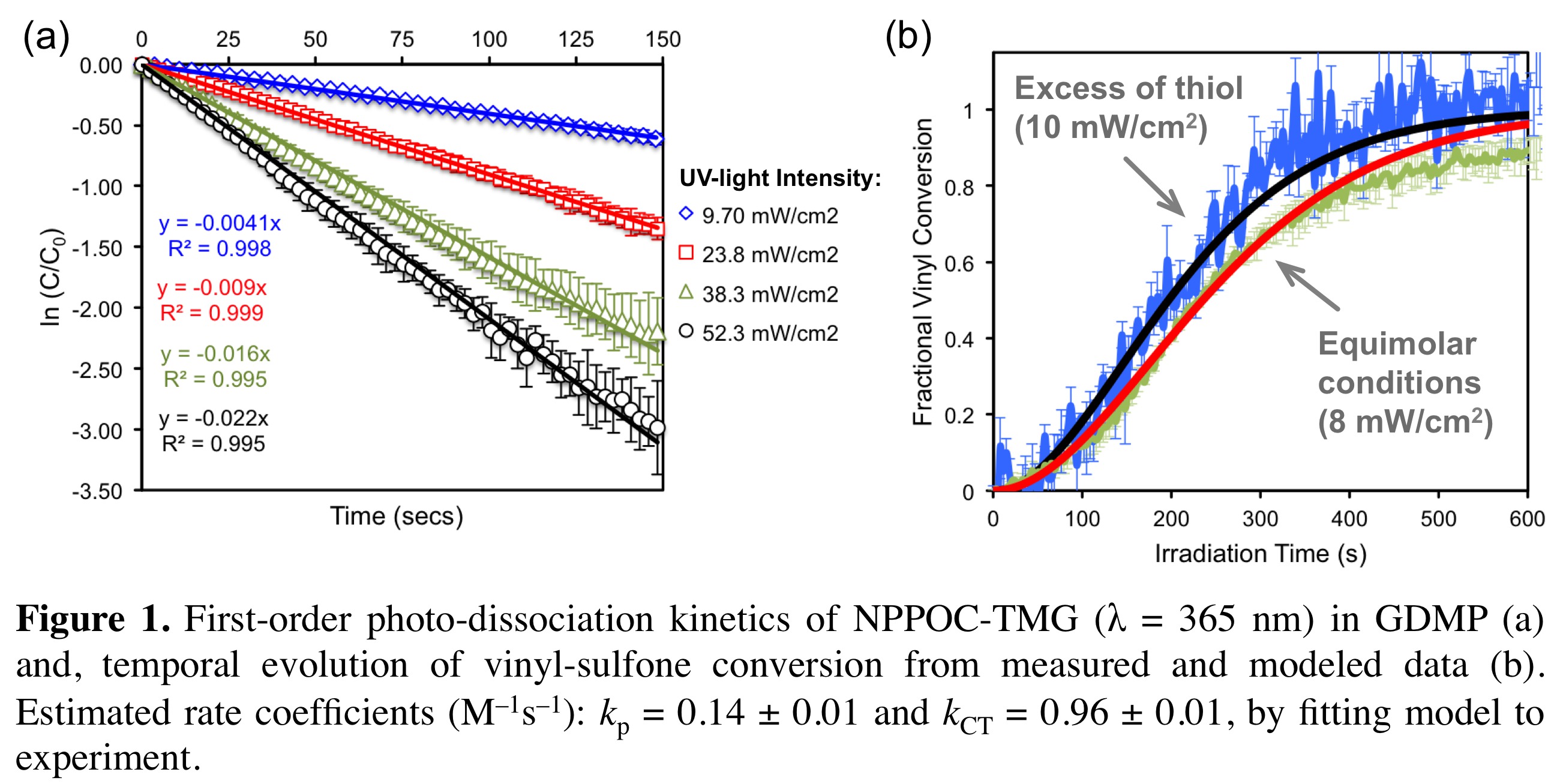
Results: Data obtained from empirical kinetics using GDMP (glycol di(3-mercaptopropionate)) and EVS (ethyl vinyl sulfone) in the presence of NPPOC-TMG (2-(2-nitrophenyl)propyloxycarbonyl/1,1,3,3-tetramethyl-guanidine, 2.0 wt.%) as superbase photo-generator, revealed a first-order dependence of the overall reaction rate on the vinyl concentration, indicating that addition of the thiolate anion to the double-bond is the rate-limiting step. Monomolecular dissociation kinetics of photobase was demonstrated with a photolysis quantum yield of φ λ(365 nm) = 0.24 ± 0.01 (Fig 1a). An estimate for the propagation/chain-transfer rate constant quotient, kp/kCT = 0.15 (Fig. 1b), was obtained by simultaneously fitting the detailed mechanism to measured kinetic data collected over a range of experimental conditions.
Conclusions: A general kinetic model was developed describing the thiol–Michael addition reaction involving a photolabile superbase catalyst. Kinetic coefficients extracted from model compound studies using mono- and di-functional thiol/vinyl-sulfone monomers were utilized to model an equivalent multifunctional photopolymerizable system. The pure step-growth nature of this living polymerization and kinetics exhibited is expected to provide more uniform cross-linked networks when compared to conventional dental systems based on dimethacrylate resins, as well as the unique potential for extended dark-cure reactions.
Methods: Kinetic studies were conducted by monitoring the progress of the photochemical reaction via time-resolved FT–IR spectroscopy and the observed kinetics modeled both analytically and numerically using a basic mechanistic scheme proposed for the reaction. Rate coefficients intrinsic to the propagation–chain-transfer mechanism, characterizing the anionic cycle, were estimated using the ‘flooding’ technique. Multifunctional photopolymerization systems were modeled using derived rate equations accounting both for the kinetics and diffusional processes.
Results: Data obtained from empirical kinetics using GDMP (glycol di(3-mercaptopropionate)) and EVS (ethyl vinyl sulfone) in the presence of NPPOC-TMG (2-(2-nitrophenyl)propyloxycarbonyl/1,1,3,3-tetramethyl-guanidine, 2.0 wt.%) as superbase photo-generator, revealed a first-order dependence of the overall reaction rate on the vinyl concentration, indicating that addition of the thiolate anion to the double-bond is the rate-limiting step. Monomolecular dissociation kinetics of photobase was demonstrated with a photolysis quantum yield of φ λ(365 nm) = 0.24 ± 0.01 (Fig 1a). An estimate for the propagation/chain-transfer rate constant quotient, kp/kCT = 0.15 (Fig. 1b), was obtained by simultaneously fitting the detailed mechanism to measured kinetic data collected over a range of experimental conditions.
Conclusions: A general kinetic model was developed describing the thiol–Michael addition reaction involving a photolabile superbase catalyst. Kinetic coefficients extracted from model compound studies using mono- and di-functional thiol/vinyl-sulfone monomers were utilized to model an equivalent multifunctional photopolymerizable system. The pure step-growth nature of this living polymerization and kinetics exhibited is expected to provide more uniform cross-linked networks when compared to conventional dental systems based on dimethacrylate resins, as well as the unique potential for extended dark-cure reactions.