IADR Abstract Archives
Degradation Resistance and Biocompatibility of Novel Multi-Functional Acrylamides
Objectives: (Meth)acrylamides have been proposed as ester-free alternatives to (meth)acrylates. The objective of this study is to evaluate novel multi-functional acrylamides in terms of resistance to degradation and biocompatibility.
Methods: Hydroxyethyl methacrylate (HEMA), N,N’-Diethyl-1,3-bis(acrylamido)propane (DEBAAP) and novel multi-functional acrylamides N,N’-bis[(3- methylaminoacryl)propyl] methylamine (BMAAPMA), tris[(2-methylaminoacryl)ethyl]amine (TMAAEA), and N,N’-bis(acrylamido) 1,4-diazepane (BAADA) were dissolved in methanol (50 mM) followed by dilution (1:100) with either acidic water (pH 1), phosphate buffered saline (PBS), or PBS + 2 units/mL each of cholesterol esterase (CE) and pseudocholinesterase (PCE). Samples were incubated for 5 days (water: RT; PBS 37°C). Remaining intact monomer concentration post-incubation was determined by HPLC (Shimadzu LC-2030, 210 nm). OD-21 cell viability was tested in serial dilutions (10 to 0.05 mM) using MTT assay. Monomers were mixed with 60 wt% UDMA, 0.2/0.4 wt% 2,2-dimethoxy-2-phenylacetophenone/DPI-PF6 (initiators), and 0.1 wt% BHT Inhibitors), and photopolymerized into discs. Final conversion (DC) was assessed in NIR prior to incubation in ethanol for 2 days. The extracted compounds were quantified by 1H NMR using 4-fluoro-2-nitrobenzamide as the internal standard. Results were analyzed with one-way ANOVA/Tukey’s test (α=0.05).
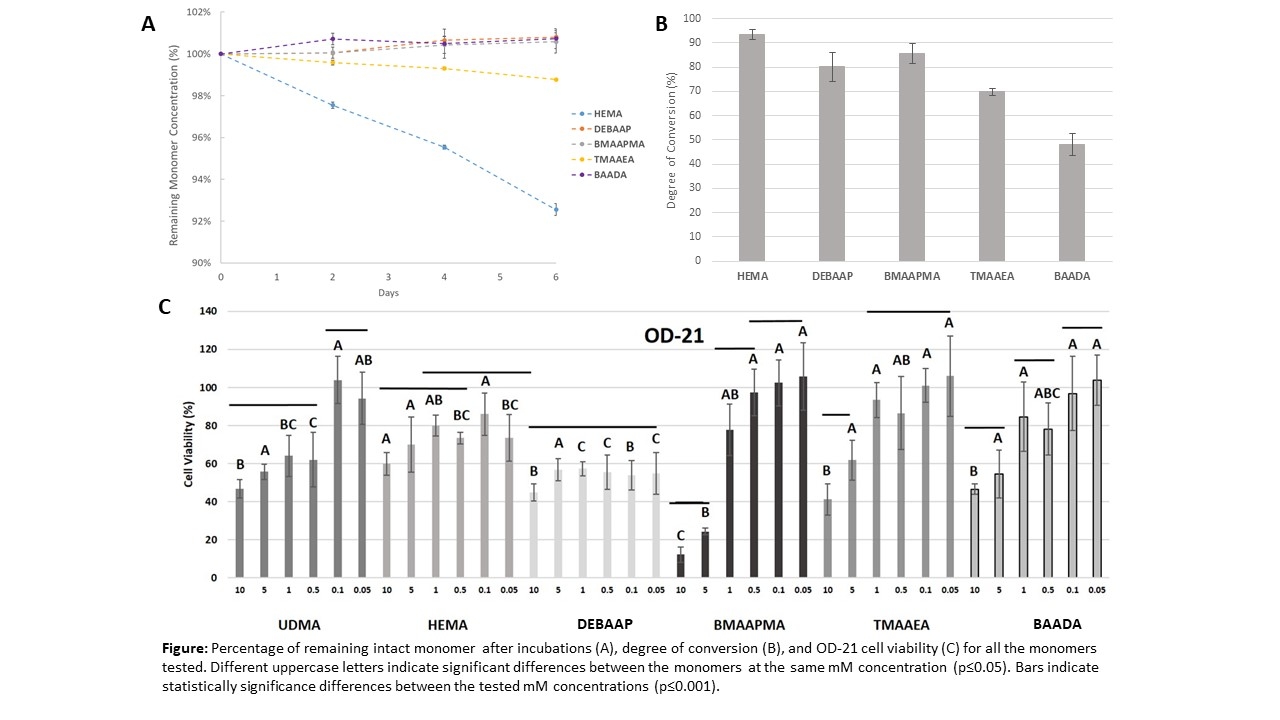
Results: Results are shown in the Figure. The multifunctional acrylamides showed minimal or no degradation in all conditions, especially BMAAPMA, DEBAAP, and BAADA. TMAAEA showed slight degradation but much less than the methacrylate control, HEMA. BAADA showed the lowest conversion, followed by TMAAEA. All others had similar conversion. Cytotoxicity was observed only at high concentrations. All monomers had at least 80% cell viability up to 0.5 mM, higher than the maximum concentration of unreacted monomers extracted after ethanol incubation.
Conclusions: The novel diacrylamide monomers showed improved resistance to degradation compared to a methacrylate control in both acidic conditions and in the presence of enzymes. OD-21 cells showed similar survival for all monomers tested at concentrations determined relevant by leachate analysis of unreacted monomers.
Methods: Hydroxyethyl methacrylate (HEMA), N,N’-Diethyl-1,3-bis(acrylamido)propane (DEBAAP) and novel multi-functional acrylamides N,N’-bis[(3- methylaminoacryl)propyl] methylamine (BMAAPMA), tris[(2-methylaminoacryl)ethyl]amine (TMAAEA), and N,N’-bis(acrylamido) 1,4-diazepane (BAADA) were dissolved in methanol (50 mM) followed by dilution (1:100) with either acidic water (pH 1), phosphate buffered saline (PBS), or PBS + 2 units/mL each of cholesterol esterase (CE) and pseudocholinesterase (PCE). Samples were incubated for 5 days (water: RT; PBS 37°C). Remaining intact monomer concentration post-incubation was determined by HPLC (Shimadzu LC-2030, 210 nm). OD-21 cell viability was tested in serial dilutions (10 to 0.05 mM) using MTT assay. Monomers were mixed with 60 wt% UDMA, 0.2/0.4 wt% 2,2-dimethoxy-2-phenylacetophenone/DPI-PF6 (initiators), and 0.1 wt% BHT Inhibitors), and photopolymerized into discs. Final conversion (DC) was assessed in NIR prior to incubation in ethanol for 2 days. The extracted compounds were quantified by 1H NMR using 4-fluoro-2-nitrobenzamide as the internal standard. Results were analyzed with one-way ANOVA/Tukey’s test (α=0.05).
Results: Results are shown in the Figure. The multifunctional acrylamides showed minimal or no degradation in all conditions, especially BMAAPMA, DEBAAP, and BAADA. TMAAEA showed slight degradation but much less than the methacrylate control, HEMA. BAADA showed the lowest conversion, followed by TMAAEA. All others had similar conversion. Cytotoxicity was observed only at high concentrations. All monomers had at least 80% cell viability up to 0.5 mM, higher than the maximum concentration of unreacted monomers extracted after ethanol incubation.
Conclusions: The novel diacrylamide monomers showed improved resistance to degradation compared to a methacrylate control in both acidic conditions and in the presence of enzymes. OD-21 cells showed similar survival for all monomers tested at concentrations determined relevant by leachate analysis of unreacted monomers.