IADR Abstract Archives
A Novel, BisGMA-free Dimethacrylate Monomer for Resin-based-composite and Adhesive Applications
Objectives: The organic matrix of dental composites and adhesives is still based primarily on BisGMA, which may cause adverse biological reactions from small molecule leachates. The aim of this study was to introduce a newly designed mixture of dimethacrylate isomers – PG6EMA, as a potential base monomer for BisGMA-free formulations.
Methods: PG6EMA was synthesized by the reaction between 4-piperidinemethanol, BPA diglycidyl ether and methacryloyl chloride. Serial amounts of ethanol (3, 6 and 9wt%) were added to the homopolymer containing DMPA photoinitiator (0.2wt%). Photocuring was carried out with a mercury arc lamp (320-500nm, 500 mW/cm2; Acticure). Neat resins or composites, formulated using either PG6EMA or UDMA mixed with TEGDMA (40:60 monomer mass ratio, 70wt% barium filler), were tested for viscosity, kinetics, wet/dry flexural strength/modulus, and water stability, as applicable. Data were analyzed with one-way ANOVA/Tukey’s and Student’s t-test (α=0.05).
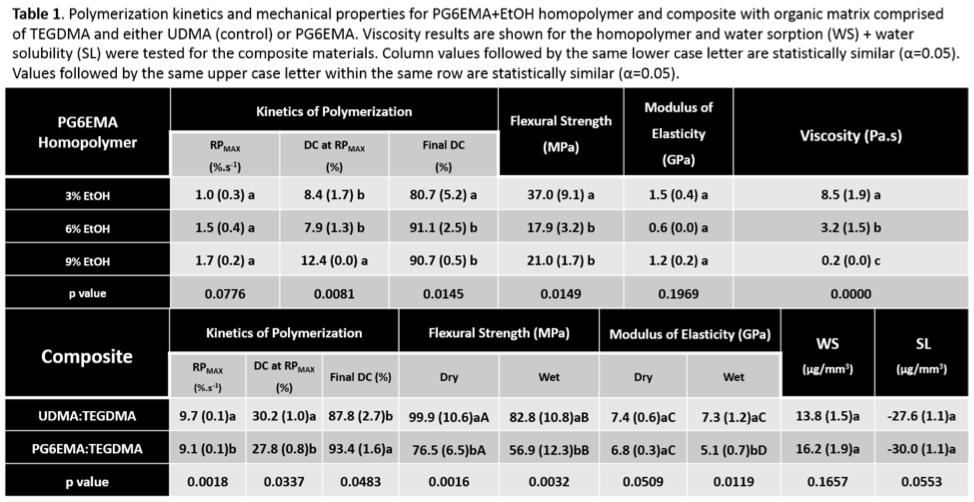
Results: All homopolymer groups showed high degrees of conversion, and varying the solvent content did not affect RPMAX(Table 1). The 9% EtOH mixture showed statistically higher DC at RPMAX and both 6% and 9% mixtures showed higher final DC, which may be a result of the systematic reduction in viscosity observed. Flexural strength of 3%EtOH homopolymer was greater than 6% and 9%, but there was no statistical difference in elastic modulus among the groups. Composites modified with PG6EMA presented slower polymerization rates but higher final DC than the control. However, the increase in conversion did not translate into enhanced mechanical properties, as PG6EMA:TEGDMA showed lower dry and wet flexural strength and comparable dry modulus. The wet modulus decrease of 25% was surprising, given the similar performance in water sorption and solubility among the composites.
Conclusions: This study demonstrates the utility of PG6EMA as a potential alternative to BisGMA for dental restorative and adhesive applications.
Methods: PG6EMA was synthesized by the reaction between 4-piperidinemethanol, BPA diglycidyl ether and methacryloyl chloride. Serial amounts of ethanol (3, 6 and 9wt%) were added to the homopolymer containing DMPA photoinitiator (0.2wt%). Photocuring was carried out with a mercury arc lamp (320-500nm, 500 mW/cm2; Acticure). Neat resins or composites, formulated using either PG6EMA or UDMA mixed with TEGDMA (40:60 monomer mass ratio, 70wt% barium filler), were tested for viscosity, kinetics, wet/dry flexural strength/modulus, and water stability, as applicable. Data were analyzed with one-way ANOVA/Tukey’s and Student’s t-test (α=0.05).
Results: All homopolymer groups showed high degrees of conversion, and varying the solvent content did not affect RPMAX(Table 1). The 9% EtOH mixture showed statistically higher DC at RPMAX and both 6% and 9% mixtures showed higher final DC, which may be a result of the systematic reduction in viscosity observed. Flexural strength of 3%EtOH homopolymer was greater than 6% and 9%, but there was no statistical difference in elastic modulus among the groups. Composites modified with PG6EMA presented slower polymerization rates but higher final DC than the control. However, the increase in conversion did not translate into enhanced mechanical properties, as PG6EMA:TEGDMA showed lower dry and wet flexural strength and comparable dry modulus. The wet modulus decrease of 25% was surprising, given the similar performance in water sorption and solubility among the composites.
Conclusions: This study demonstrates the utility of PG6EMA as a potential alternative to BisGMA for dental restorative and adhesive applications.