IADR Abstract Archives
Reporting of Adverse Reactions to Dental Biomaterials 1993-2018 in Norway
Objectives: For more than 25 years, the Norwegian Dental Biomaterials Adverse Reaction Unit has been responsible for a national reporting procedure concerning suspected clinical biologic adverse reactions associated with dental biomaterials. The procedure is based on spontaneous and voluntary reporting from dentists, physicians and dental hygienists. The reporting is designed to record all types of adverse reactions and is not limited to serious events described in the reporting procedures based on the European Medical Device Regulation (MDR). From January 2008, the use of dental amalgam, along with other mercury-containing products, was heavily restricted, and later prohibited (December 2010) by Norwegian authorities due to environmental concerns. The objectives of the adverse reaction reporting are to gain knowledge about material-associated adverse reactions and to monitor changes over time.
Methods: Reporting forms are available from the Norwegian Dental Journal, from the internet pages of Norwegian Dental Biomaterials Adverse Reaction Unit, and from the most commonly used electronic patient record software in Norway. Adverse reaction reports were registered in a database and analyzed with regard to the types of materials involved using multiple response analysis on a yearly basis. The following categories were analyzed: dental amalgam, composites and cements, metals and alloys, and materials for short-term use.
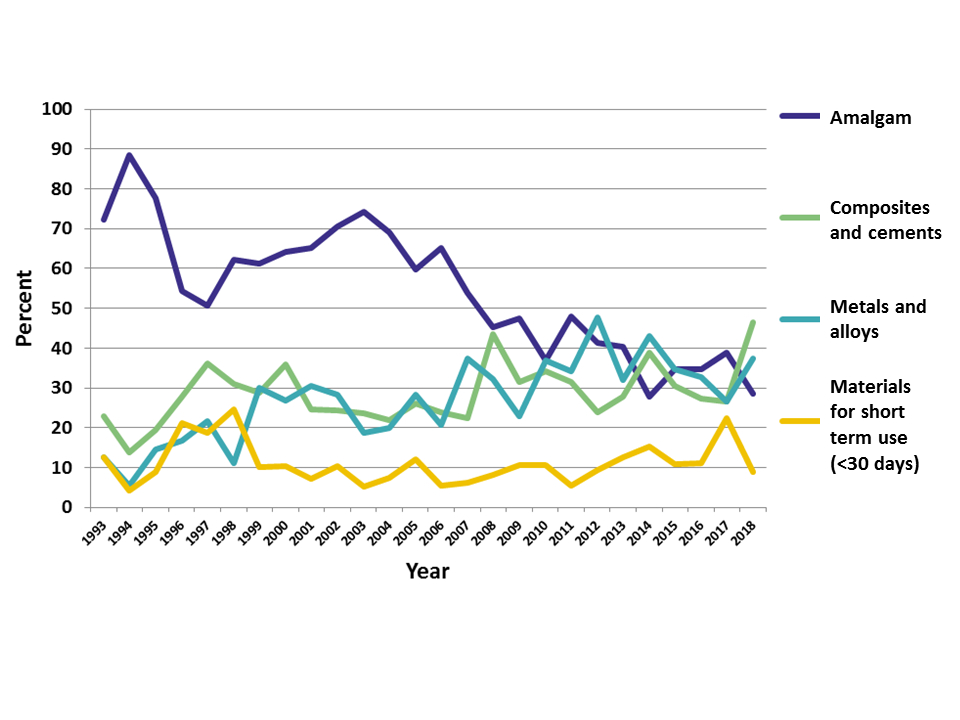
Results: From 1993 to the end of 2018, 2523 reports were received. The proportion of reports related to amalgam has decreased over time. In 2018 it was 28.6 % and lower than the proportion of reports related to metals/alloys (37.5 %), and composites/cements (46.4 %; Figure).
Conclusions: The proportion of amalgam related reports has decreased considerably from 1993 to 2018. The proportion of reports regarding composites and cements has increased marginally the same period. Since data are based on reports concerning suspected reactions, detailed clinical investigations of the patients are needed to assess possible causal relationships.
Methods: Reporting forms are available from the Norwegian Dental Journal, from the internet pages of Norwegian Dental Biomaterials Adverse Reaction Unit, and from the most commonly used electronic patient record software in Norway. Adverse reaction reports were registered in a database and analyzed with regard to the types of materials involved using multiple response analysis on a yearly basis. The following categories were analyzed: dental amalgam, composites and cements, metals and alloys, and materials for short-term use.
Results: From 1993 to the end of 2018, 2523 reports were received. The proportion of reports related to amalgam has decreased over time. In 2018 it was 28.6 % and lower than the proportion of reports related to metals/alloys (37.5 %), and composites/cements (46.4 %; Figure).
Conclusions: The proportion of amalgam related reports has decreased considerably from 1993 to 2018. The proportion of reports regarding composites and cements has increased marginally the same period. Since data are based on reports concerning suspected reactions, detailed clinical investigations of the patients are needed to assess possible causal relationships.