IADR Abstract Archives
The Safety and Efficacy of "Over The Counter” Bleaching Products in the United Kingdom
Objectives: To determine the safety and efficacy of non hydrogen peroxide OTC whitening products available in the United Kingdom.
Methods: A total of 21 extracted teeth (11 incisors and 10 premolars) were collected and stored in chloramine T solution. 5 days prior to the study, all teeth where was immersed in 5 ml of a standard green tea solution at room temperature (22±2 °C). Roots were sectioned from the teeth and cleaned using an ultrasonic bath. Teeth were then embedded in epoxy resin and sectioned inciso-gingivally to serve as paired test and control specimens. 10% Carbamide peroxide was used as a positive control and saline as a negative control. Five OTC products were selected from two major British consumer outlets. Initially, products were applied to the teeth samples for 2x 1 hour cycles, followed by equivalent of one week application according to manufacturer’s instruction. Samples were stored overnight in saline to minimise any effects of dehydration. Shade of teeth were taken blindly by a single trained clinician in a natural light environment against a grey background prior and post applicaiton of the products. Vickers microhardness tests and SEM analysis was undertaken. Vickers microhardness of the experimental and paired control halves were statistically compared to the baseline values using Wilcoxon-tests (p<0.05).
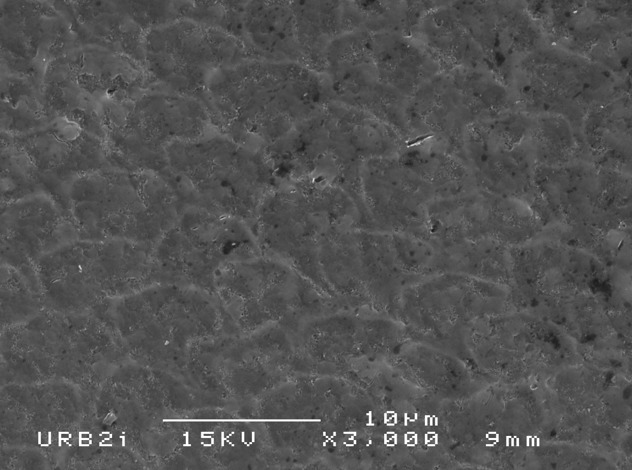
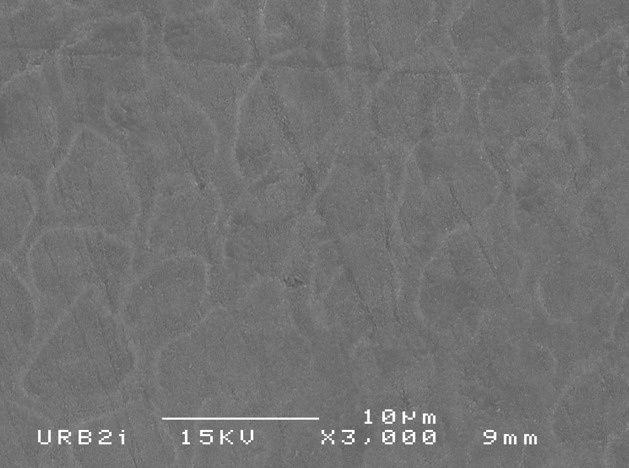
Results: SEM analysis revealed surface morphology alterations to varying degrees with several samples demonstrating a distinct etching pattern post exposure to the OTC products. Sample 3 (Brilliant 5 minute kit) and sample 5 (iWhite instant teeth whitening) produced the most extensive surface alterations. Sample 3 and 5 also resulted in a significant reduction (p=0.008) in Vickers microhardness. Two OTC products resulted in a lightening effect less than the negative control saline, whereas two OTC resulted in a lightening effect greater than CP
Conclusions: This study suggests that non hydrogen peroxide OTC products have the potential to damage enamel and the potential to lighten teeth. The lightening effect of the OTC products is variable however is most likely to occur in sodium chlorite based products
Methods: A total of 21 extracted teeth (11 incisors and 10 premolars) were collected and stored in chloramine T solution. 5 days prior to the study, all teeth where was immersed in 5 ml of a standard green tea solution at room temperature (22±2 °C). Roots were sectioned from the teeth and cleaned using an ultrasonic bath. Teeth were then embedded in epoxy resin and sectioned inciso-gingivally to serve as paired test and control specimens. 10% Carbamide peroxide was used as a positive control and saline as a negative control. Five OTC products were selected from two major British consumer outlets. Initially, products were applied to the teeth samples for 2x 1 hour cycles, followed by equivalent of one week application according to manufacturer’s instruction. Samples were stored overnight in saline to minimise any effects of dehydration. Shade of teeth were taken blindly by a single trained clinician in a natural light environment against a grey background prior and post applicaiton of the products. Vickers microhardness tests and SEM analysis was undertaken. Vickers microhardness of the experimental and paired control halves were statistically compared to the baseline values using Wilcoxon-tests (p<0.05).
Results: SEM analysis revealed surface morphology alterations to varying degrees with several samples demonstrating a distinct etching pattern post exposure to the OTC products. Sample 3 (Brilliant 5 minute kit) and sample 5 (iWhite instant teeth whitening) produced the most extensive surface alterations. Sample 3 and 5 also resulted in a significant reduction (p=0.008) in Vickers microhardness. Two OTC products resulted in a lightening effect less than the negative control saline, whereas two OTC resulted in a lightening effect greater than CP
Conclusions: This study suggests that non hydrogen peroxide OTC products have the potential to damage enamel and the potential to lighten teeth. The lightening effect of the OTC products is variable however is most likely to occur in sodium chlorite based products