IADR Abstract Archives
Periostin Modification is Essential for Supereruption and Collagen Matrix Architecture
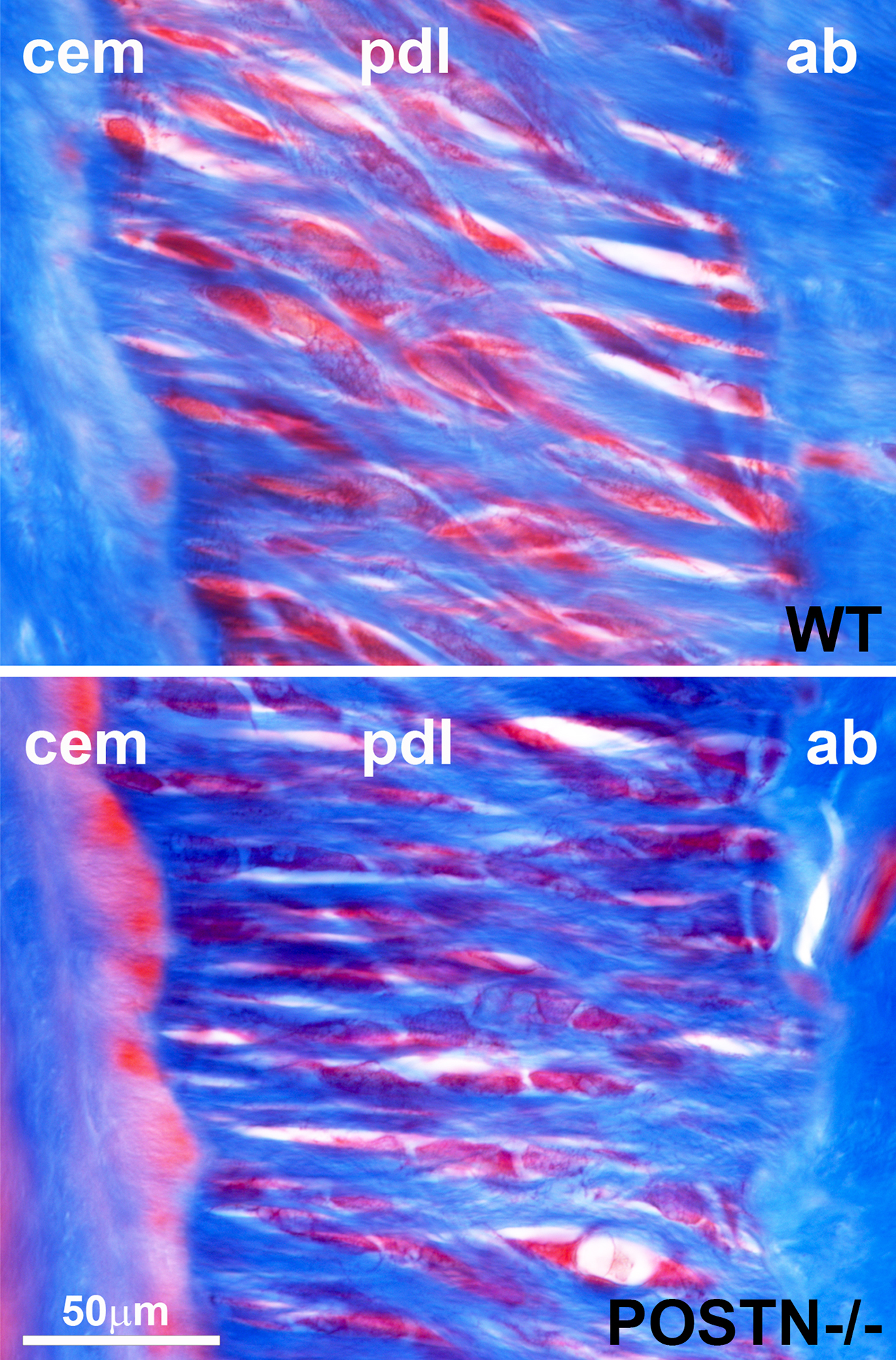
Objectives: Periostin (POSTN) is a matricellular protein predominantly expressed in collagen-rich fibrous connective tissues such as periosteum, perichondrium, and periodontal ligament. Previous studies have proposed that periostin might play an essential role for periodontal ligament during occlusal loading. To determine the effect of POSTN on collagen fiber assembly and dimensions as a mechanism affecting periodontal ligament (PDL) elasticity and resilience.
Methods: Sharpey’s fiber bundle diameter was compared between POSTN null and wild-type mice, and the effect of POSTN on the resilience of the PDL fiber apparatus following occlusal unloading was assessed using the un-opposed molar model. To determine the effect of POSTN on collagen assembly, collagen/POSTN composite sponges were compared to collagen sponges alone using atomic force microscope, circular dichroism assays, infrared spectroscopy, X-ray diffraction, and Pyridinoline cross-linking assays.
Results: The periodontal collagen fiber volume fraction was 4.7-fold increased in POSTN null mice when compared to wild-type controls. Loss of occlusal loading in the un-opposed molar model abolished super-eruption and resulted in a 1-2µm decrease in occlusal height versus a 130µm super-eruption in control mice. Collagen/POSTN composite sponges vs. control collagen sponges demonstrated a loss in triple helical collagen fibers as revealed by atomic force micrographs, a conformational change as identified by infrared spectroscopy and circular dichroism studies, and a loss of periodic diffraction rings in X-ray diffraction comparisons. Moreover, there was a highly significant 1.9-fold in Pyridinoline cross-links when POSTN was added to the collagen fiber sponge matrix composition.
Conclusions: Our study demonstrated that POSTN was required for tooth supereruption following occlusal unloading. Lack of periodontal fiber resilience in POSTN null mice was a result of dramatic changes in collagen architecture, including an increase in fiber diameter, a reduction in triple helical organization, a reduction in cross-linking, and an overall conformational change in collagen three-dimensional organization.
Methods: Sharpey’s fiber bundle diameter was compared between POSTN null and wild-type mice, and the effect of POSTN on the resilience of the PDL fiber apparatus following occlusal unloading was assessed using the un-opposed molar model. To determine the effect of POSTN on collagen assembly, collagen/POSTN composite sponges were compared to collagen sponges alone using atomic force microscope, circular dichroism assays, infrared spectroscopy, X-ray diffraction, and Pyridinoline cross-linking assays.
Results: The periodontal collagen fiber volume fraction was 4.7-fold increased in POSTN null mice when compared to wild-type controls. Loss of occlusal loading in the un-opposed molar model abolished super-eruption and resulted in a 1-2µm decrease in occlusal height versus a 130µm super-eruption in control mice. Collagen/POSTN composite sponges vs. control collagen sponges demonstrated a loss in triple helical collagen fibers as revealed by atomic force micrographs, a conformational change as identified by infrared spectroscopy and circular dichroism studies, and a loss of periodic diffraction rings in X-ray diffraction comparisons. Moreover, there was a highly significant 1.9-fold in Pyridinoline cross-links when POSTN was added to the collagen fiber sponge matrix composition.
Conclusions: Our study demonstrated that POSTN was required for tooth supereruption following occlusal unloading. Lack of periodontal fiber resilience in POSTN null mice was a result of dramatic changes in collagen architecture, including an increase in fiber diameter, a reduction in triple helical organization, a reduction in cross-linking, and an overall conformational change in collagen three-dimensional organization.