IADR Abstract Archives
Treatment Guidelines for Narrow Diameter Implant(NDI)
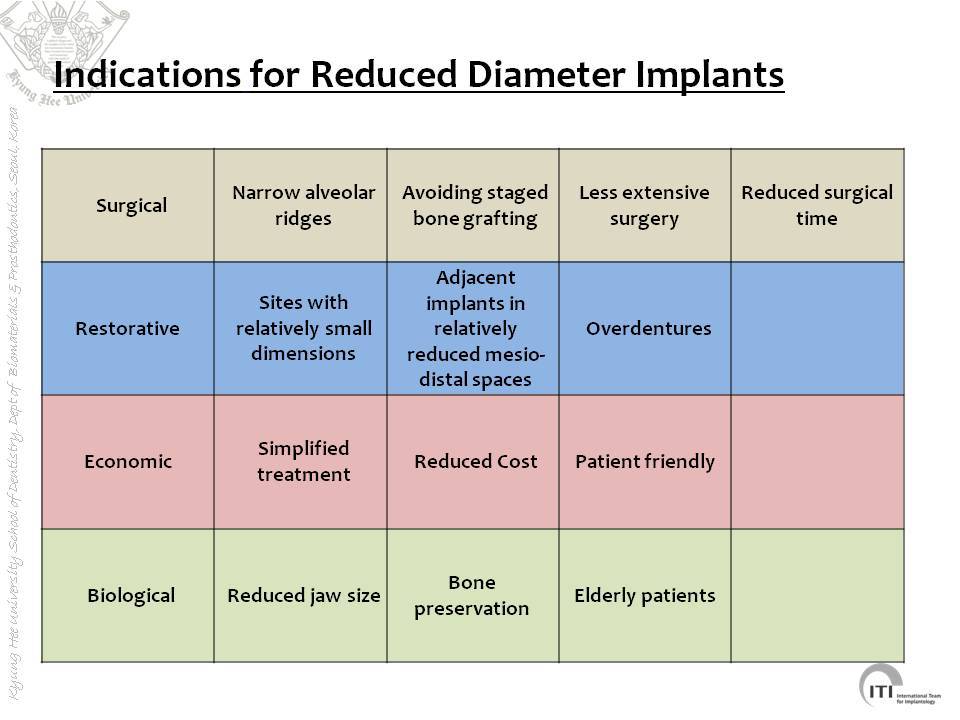
Objectives: Narrow (reduced)-diameter implants (NDI) were categorized as follows for the systematic review by Klein et al: Category 1: one-piece, < 3.0 mm (mini-implants), Category 2: two-piece, 3.00 to 3.25 mm, and Category 3: two-piece, 3.30 to 3.50 mm. Potential benefits of NDIs may include less invasive surgery and reduced need for bone augmentation. However, these issues have not yet been scientifically assessed. Possible confounding factors of the studies evaluated might be available bone quantity and quality, splinting of the suprastructure, loading forces of the opposing dentition, and the biomechanical role of the implant-abutment connection.
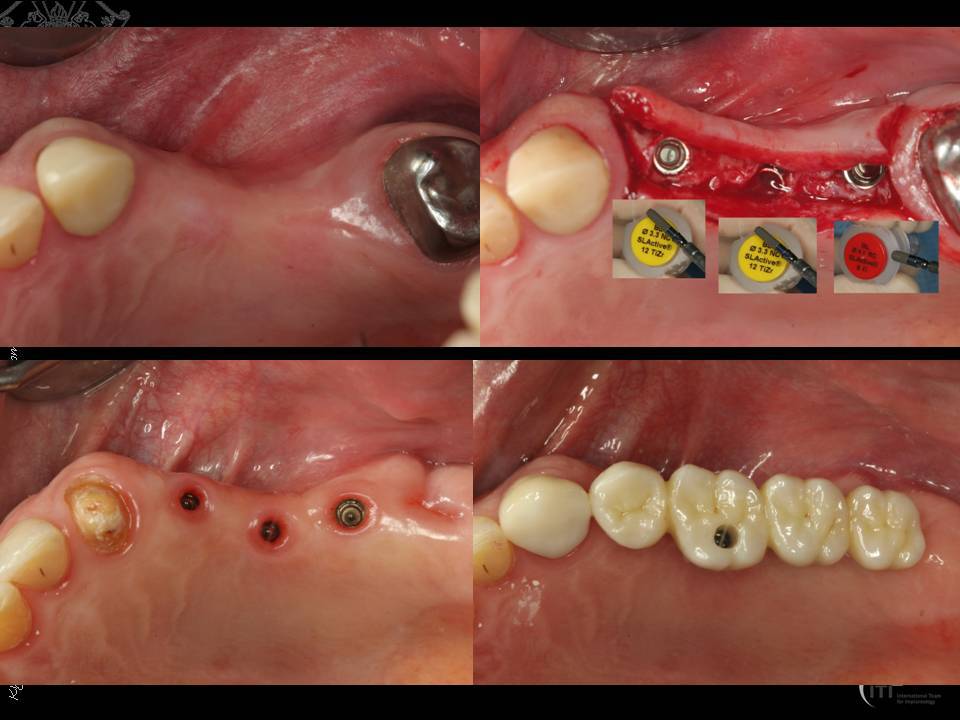
Methods: NDIs were placed in situations with reduced mesiodistal space or reduced ridge width, provided that the general positioning rules are followed. Clinical studies on dental implant survival under functional loading as well as radiographic analysis of the marginal bone level on 10 treated patients were evaluated.
Results: 1) Two-piece titanium implants with a diameter of 3.3 to 3.5 mm demonstrated a mean survival rate 96.9% (89% to 100%) after a mean follow-up time of 4.1 years (1 to 11 years) for all indications including posterior regions.
2) There is insufficient evidence on the success rates for all NDIs. Clinical parameters and treatment protocols are often not sufficiently described and no controlled comparative studies are available, resulting in a high risk of bias.
Conclusions: Clinical indications may include: 1) Single-tooth replacements in the anterior zones: categories 1, 2, and 3 (category 1 and 2 only for incisors). One-piece implants often have specific prosthodontic disadvantages. 2) Edentulous jaws to be rehabilitated with overdentures: categories 2 and 3, and category 1 for mandibles only (4 implants). 3) Single posterior, multiple-unit fixed dental prothesis(FDP), and edentulous jaws to be rehabilitated with FDP: only category 3; individual informed consent should include the possibility of more technical complications. Alternative treatment options should also be discussed.
Methods: NDIs were placed in situations with reduced mesiodistal space or reduced ridge width, provided that the general positioning rules are followed. Clinical studies on dental implant survival under functional loading as well as radiographic analysis of the marginal bone level on 10 treated patients were evaluated.
Results: 1) Two-piece titanium implants with a diameter of 3.3 to 3.5 mm demonstrated a mean survival rate 96.9% (89% to 100%) after a mean follow-up time of 4.1 years (1 to 11 years) for all indications including posterior regions.
2) There is insufficient evidence on the success rates for all NDIs. Clinical parameters and treatment protocols are often not sufficiently described and no controlled comparative studies are available, resulting in a high risk of bias.
Conclusions: Clinical indications may include: 1) Single-tooth replacements in the anterior zones: categories 1, 2, and 3 (category 1 and 2 only for incisors). One-piece implants often have specific prosthodontic disadvantages. 2) Edentulous jaws to be rehabilitated with overdentures: categories 2 and 3, and category 1 for mandibles only (4 implants). 3) Single posterior, multiple-unit fixed dental prothesis(FDP), and edentulous jaws to be rehabilitated with FDP: only category 3; individual informed consent should include the possibility of more technical complications. Alternative treatment options should also be discussed.